About us
Indian Medical Device, IVD manufacturers and importers are required to comply with CDSCO Medical Device Rules 2017 (popularly known as MDR 2017) and its Amendments thereafter irrespective of the global market requirements. The regulation got structured in 2017 and is now expanding to have every medical device under it through given timelines. These Rules are quite integrated with global regulation of medical devices. The Rules cover product’s life cycle and are to be implemented through ISO13485 based Quality Management System. The compliance to Rules focusses on manufacturer’s intent, infrastructure, processes, technology and people based on classification of medical devices. Intrust-Consulting helps Client’s Team to Conceptualize, Build, Implement and Operate a Quality Management System fulfilling all Regulatory requirements. Our approach of consultancy is simple, easy to understand, inculcate and is enabling your team for future. Our consultancy leads to a well-designed “Integrated Quality Management System” which becomes one step solution for your global compliance corporate Quality Objective. Once implemented your registrations, accreditations come with ease. Our Quality Management System and Regulatory consultancy helps your team Master the Basics, Improve & Build Capacity to achieve Product & System Performance Excellence.
Misson
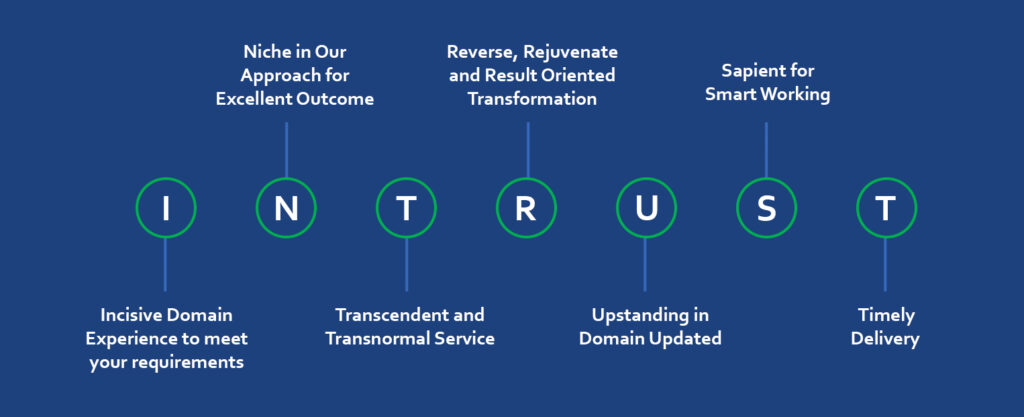
Inform, Reform and Transform the QMS through cross functional involvement during consultancy period.
Vision
Our client shall achieve the TRUST of Regulation IN Medical Device & In Vitro Diagnostic Devices.
Niche
Techno-QMS-Regulatory experience in MD & IVD Domain (Cumulative Working Experience of >50 years)
Inspired by Global Experience & Fueled by Niche Intent.
Our Team
Our Team consists of several members having domain experience from Medical Device & IVD industry. Their cumulative experience runs into several decades and rich to handle all queries and projects.

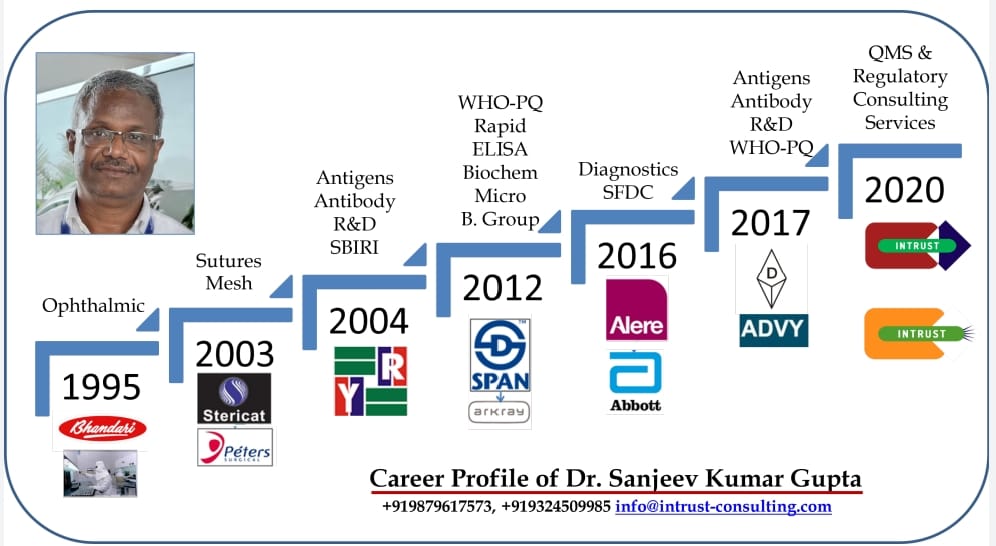
Dr. Sanjeev Kumar Gupta
(Managing Consultant)
Dr. Sanjeev has worked in several national and international medical device companies for 25 years. He has strong domain experience from Medical Device (all classes) & IVD (all classes) industry which covers product life cycle from customer requirement to post market surveillance. He designed, implemented and monitored ISO 13485 & ISO 9001 based QMS with in-built regulatory requirements. He has worked in multi-cultural teams in multinationals as well as localized teams in national companies which enabled him to translate requirements into long lasting compliances. He is credited with first WHO-PQ of Malaria Rapid Test Product in India. In one year of existence, he has designed GMP facilities (MD, IVD, Combination Devices), designed QMS (ISO 13485, Schedule V, MDR-745 and IVDR- 746, MDSAP), trained man-power for QMS processes, established regulatory strategies at several client sites. He has trained over 100 professionals for CDSCO-MDR, 2017 in close cooperation with ADMI and AIMED. Hosted COVID19 time webinars, delivered lectures and published articles for continued industry awareness.

Urit Gupta
(Consultant & Partner)
A young dynamic partner in Intrust-Consulting who is completely focused on Quality and Regulatory aspects of Software as Medical Devices (SaMD). It is his incredible thinking behind logo of our company which depicts process approach to ISO 13485. His involvement is growing in other areas of company.

Urit Gupta
(Consultant & Partner)
A young dynamic partner in Intrust-Consulting who is completely focused on Quality and Regulatory aspects of Software as Medical Devices (SaMD). It is his incredible thinking behind logo of our company which depicts process approach to ISO 13485. His involvement is growing in other areas of company.
Advisory Board

David Harrison
David advises Intrust with his 4 decade long QA/RA experience at Abbott, Alere, Brandwood, Siemens and Bayer. He is currently associated with DWH Consulting. His command on regulatory and QMS requirements connect CFR 821, ISO 13485, TGA, Chinese and Korean regulation with a great ease. He drives us into values, passion, focus and ideas on table for us to excel.

Dr. Taruna Madan
A well-known Scientist and Innate Immunologist at ICMR she holds degrees in B.Pharm. M.Pharm and Doctorate. Her research career with development of In Vitro Diagnostic ELISA Kit for Aspergillosis, Anti-HIV gel for gynecology application, several patents including one for COVID19 adjunct therapy brings expertise to review science behind critical designs intended for human health.

Dr. Abhijit Datta
Dr. Abhijit Datta is Vice President of Operations at Diazyme Laboratories and serves as a member of executive management team. He has rich experience in Product Development and phase transition products through regulatory cycles in compliance with FDA, Health Canada, NMPA and EU: CE IVD, USDA, DEA and various local and state regulatory authorities. He was American Cancer Society Post-Doctoral Fellow at the Dana-Farber Cancer Institute, Harvard University Medical School and the Ludwig Institute of Cancer Research, UCSD. Dr. Datta has served on the board of Diazyme’s Shanghai affiliate and serves as an advisor to biotech companies.

Dr. Beena Bhatia
Dr. Beena Bhatia is currently Senior Project Manager at Foundation Medicine. She is PMP certified, highly productive manager with several years of pharmaceutical and Medical Devices experience. Dr. Bhatia is highly skilled at managing multiple projects and ensuring time-bound delivery of commitments by establishing and implementing project plans and schedules from initiation through delivery. She received the “2019 Heroes of Chemistry” award from American Chemical Society for the discovery and development of NUZYRA™ (Omadacycline), an antibacterial drug and SEYSARA® (Sarecycline), an acne drug, both approved by FDA.

Arvind Sethia
An engineer & software expert Arvind brings knowledge of consistent delivery to customers with complete satisfaction. He advises Intrust-Consulting in its endeavor to connect with latest computer and software technologies which are currently emphasized in software Medical Device and IVDs & Cyber security requirements. His vast experience from TCS, Information Mosaic, Sapient, Nucleus and Petro IT helps us in capturing diversity and specificity of customer requirement.

