Build SMART QMS
Help you Design, Implement and Manage the QMS fully customized through the extent of your Organization.
IMPROVE HEALTH OF YOUR IMPLEMENTED QMS
Services
MFG FacilityDesign / Upgrade

Clinical InvestigationClinical Performance Evaluation
QA / RegulatoryDocument Design
Preparedness forCertification
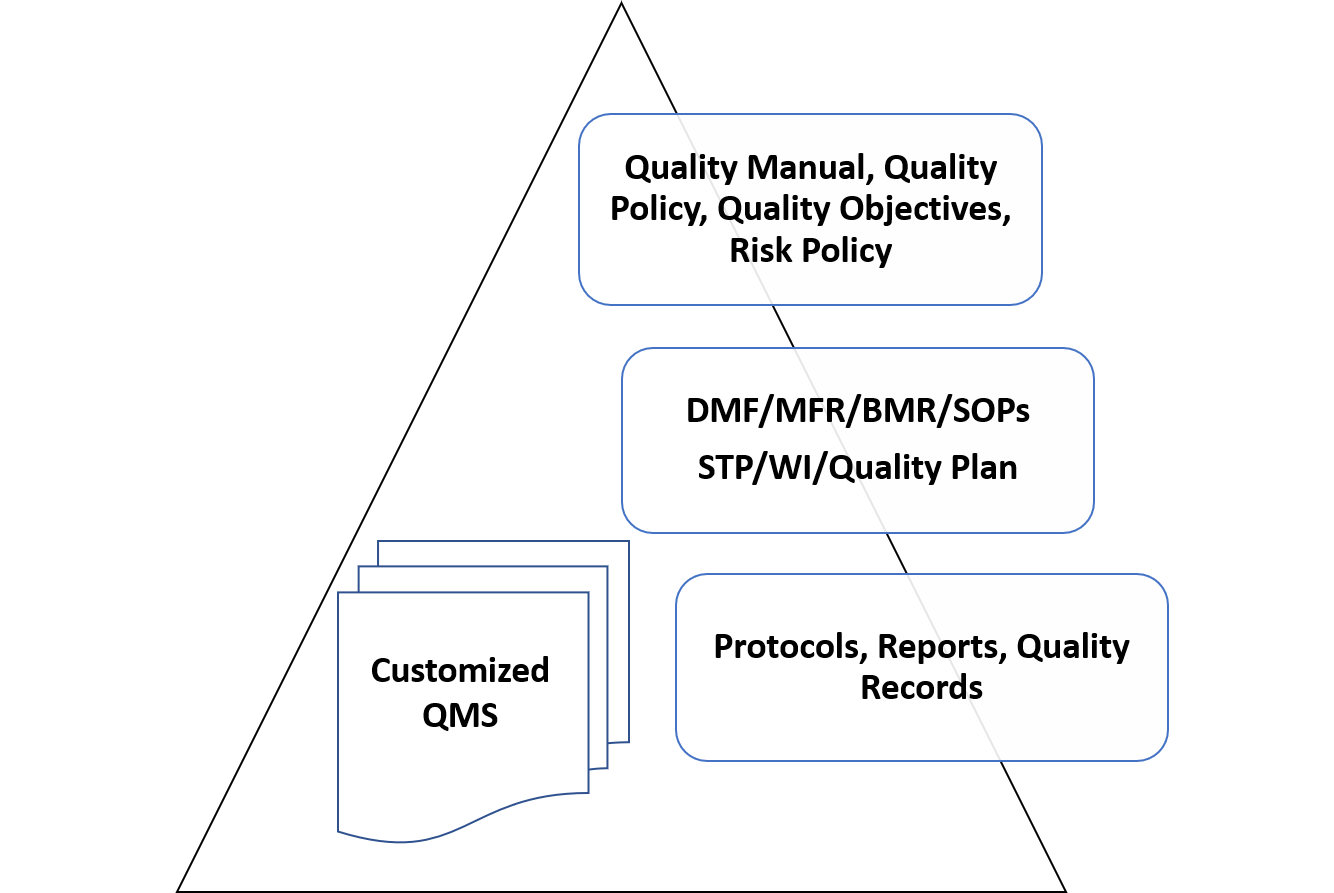
SMART Quality Management System
Intrust-Consulting helps its client to Develop an ISO 13485:2016 based SMART-QMS (Specific, Manageable, Actionable, Regulatory compliant & Total Quality Management System) which enables cross functional harmony & interdependence. The SMART-QMS helps our clients in maintaining consistent compliance and demonstration of QMS health during external audits and inspections. SMART-QMS improves itself as the need arises in a smooth way. The SMART-QMS shall comply with requirements of CDSCO-MDR 2017 & EN ISO 13485: 2016 for CE marking.

Design Verification & Validation

Clinical Investigations of Medical Devices

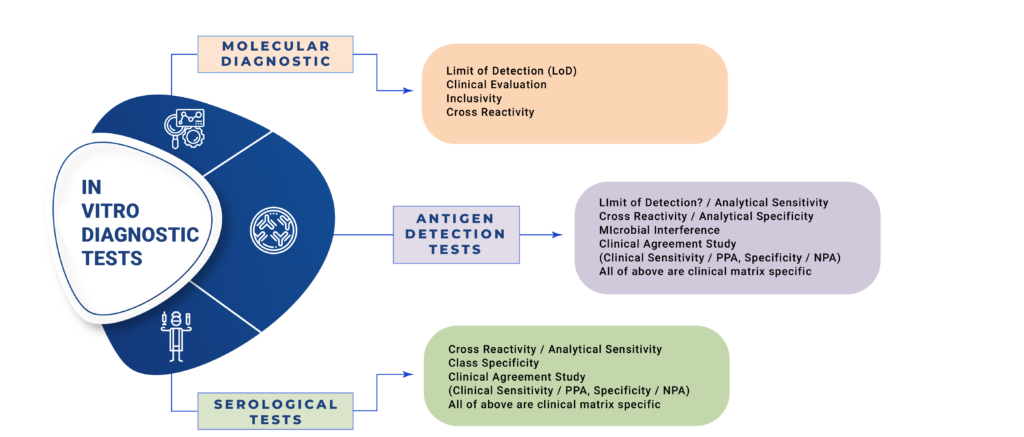
Performance Evaluation of IVD

Clinical Performance Evaluation of New IVD
Our Core Values support your Quality & Regulatory Aims
MDSAP QMS
Intrust-Consulting helps its client to achieve MDSAP-QMS (Medical Device Single Assessment Program Quality Management System) through design, implementation and monitoring. The MDSAP-QMS is a way that medical device manufacturers can be audited once for compliance with the standard and regulatory requirements of Australia (TG-MD-R Sch 3), Brazil (RDC/ANVISA 16/2013), Canada, Japan (MHLW Ordinance 169) and the United States (21CFR820). The MDSAP-QMS compliance enhances product quality and acceptance worldwide and it helps manufacturer avoid regulatory country site inspections. They can submit the MDSAP-QMS certificates for easy access to registration of manufacturing site in these countries. As of now EU is not accepting MDSAP-QMS but its not far that the MDSAP will be admitted in EU registration process.

CDSCO MDR 2017
Intrust-Consulting advises manufacturers & importers to achieve compliance to CDSCO MDR 2017 (Chapters, Rules, Schedules, Forms & Amendments) requirements through a close interaction, hand-holding and training. Every thing that fits Medical Device Definition (GSR S.O. 648(E) 11th Feb-2020) will be under direct regulation from August 11th, 2023. Our SMART-QMS and Regulatory Technical Documentation Consultancy Services align perfectly well for achieving compliance and obtaining necessary licenses and trade documents. For this we take up green field and brown field projects in this segment.
Regulatory Technical Documentation (RTD)
Intrust-Consulting with its domain experience enables its clients to inculcate Technical Documentation right from Design Stage. The technical documentation includes information of product’s design, manufacturing process, supplier, safety & performance, product’s verification & validation information, labelling & packaging, Instruction for Use (IFU) and risk management report. In addition, one needs to write host of clinical work documentation. We enable through hand-holding and review. Our RTC services enables clients to achieve compliance for CE marking as per MDR-745 and IVDR-746. Our RTC enables for Post Market Surveillance (PMS), Periodic Safety Update Reports (PSUR), Summary of Safety and Clinical Performance (SSCP) and Post Market Clinical Follow Up (PMCF)


Medical Device & In Vitro Diagnostic Device Design Validation
Intrust-Consulting imparts training & help companies for Clinical Investigations of Medical Devices, Performance Evaluation & Clinical Performance Evaluations of In Vitro Diagnostic Devices. These can be aligned with ISO standards, global and local regulatory requirements. We interact with your Design Team to ensure complete compliance for design verification and validation. We have full experience in drafting EC protocols, Informed Consent Form, Investigator Brochures, Case Record Forms, Data analysis statistics and scientific writing for Technical Files, Peer Reviewed Journals and Blogs. Biocompatibility studies for biomaterials used in medical devices as per ISO 10993. Stability studies of Sterile Packaging, In Vitro Diagnostic Kits, IVD Raw Materials. Electrical and Electronic Safety.
Trainings
Intrust-Consulting Conducts Awareness & Implementation Trainings

Regulatory Training
- CDSCO MDR 2017 & Amendments
- MDSAP
- 510k
- Clinical Evidence Report (CER)
- Post Market Clinical Follow Up (PMCF)
- CE Marking (MDR-745 & IVDR-746)
- Product registrations in different countries
QMS Process Training
- Process Approach
- Post Market Surveillance
- Vendor Audits
- Identification & Traceability
- Hazard Analysis and Risk Management
- How to respond External Audit NCs
- Technical Documentation Writing
- Management Review
- Design & Development
- Data Analysis
- Computer System Validation
- How to face Internal and External Audits
- Validation Master Plan, Validation Protocol Writing, Conducting Validations
- Good Documentation Practices
- Correction, Corrective Action
- Internal Audit
- Cleaning process
- Customer Complaint & Feedback Handling & Management
- Vendor Qualification & Assessment

QMS Process Training
Process Approach | Management Review | Internal Audit | Design & Development | Hazard Analysis and Risk Management | Vendor Qualification & Assessment | Identification & Traceability | Validation Master Plan, Validation Protocol Writing, Conducting Validations | Good Documentation Practices | Technical Documentation Writing | How to face Internal and External Audits | How to respond External Audit NCs | Post Market Surveillance | Data Analysis | Computer System Validation | Vendor Audits | Customer Complaint & Feedback Handling & Management | Correction, Corrective Action | Cleaning process
Trainings Sessions
Smart Route to Your Certification
eu mdr 745 & ivdr 746, usfda